CoLumbo
CoLumbo is an AI assistant software for the interpretation of lumbar spine MRI scans.
Overview
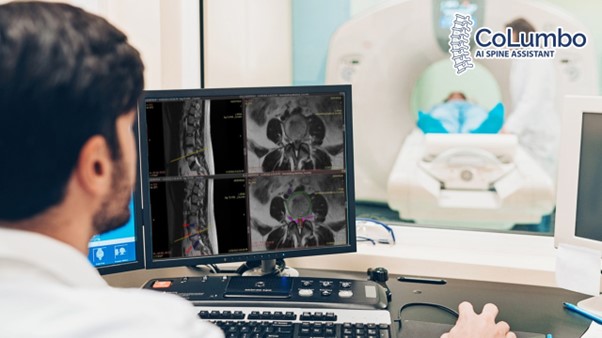
CoLumbo is an AI-based software for reading and analysis of lumbar spine MR scans. It is an assistant type of software that identifies and measures all major structures of the lumbar spine, through automatic segmentation, and provides a detailed description of findings in an editable draft report.
CoLumbo aims to help healthcare organizations resolve the following issues:
- Shortage of radiologists
- Exponential increase in number of spine MRI scans
- Disagreement between radiologists
- Lack of reporting consistency and standardization

Features
- Pre-populated reports
- Multiple measurements and findings
- Segmentations of different tissues
- Fully customizable thresholds
- Report available in English, German, French and more
Pathologies related to supported measurements:
- Herniation and its size, location and possible descending and/or ascending migration
- Protrusion and extrusion herniation modifiers
- Nerve root impingement
- Dural sac compression
- Central spinal stenosis – Schizas and Lee morphological grading
- Antero and retro – spondylolisthesis
- Symmetrical and asymmetrical bulging
- Hypo and hyper-lordosis
- Reduced disk height
- Reduced vertebral body height
Benefits
- Time savings in MRI scan reading and reporting.
- Reduced errors of omission.
- Aims to improve patient–physician communication.
- Expected to bring more consistency and standardization in spine MRI reporting.
Image examples


If you’re interested in learning more about this solution, or the 100+ other applications we have available on Blackford platform book a call with our friendly team now!

Book a meeting
We’d welcome the opportunity to learn more about your AI needs and to explain how partnering with Blackford can drive efficiency and provide ongoing value.
Book a Meeting¹Georgiev R, Novakova M, Mitev D, Cilkov D, Kostova E. Clinical Evaluation Report in Regards to the Progress of Clinical Trial Approved by Ethics Committee for Clinical Investigation of Medical Device with Statement № ЕККИ / СТ – 0687 . Valna, Bulgaria: Smart Soft LTD; p. 1–50.